How To Find Molar Mass From Moles
v.4: Tooth Mass- Mole-to-Mass and Mass-to-Mole Conversions
- Page ID
- 152164
Learning Objectives
- Perform conversions between mass and moles of a substance.
- Convert from mass or moles of one substance to mass or moles of another substance in a chemical reaction.
- Use a counterbalanced chemic equation to determine molar relationships between substances.
Molar Mass
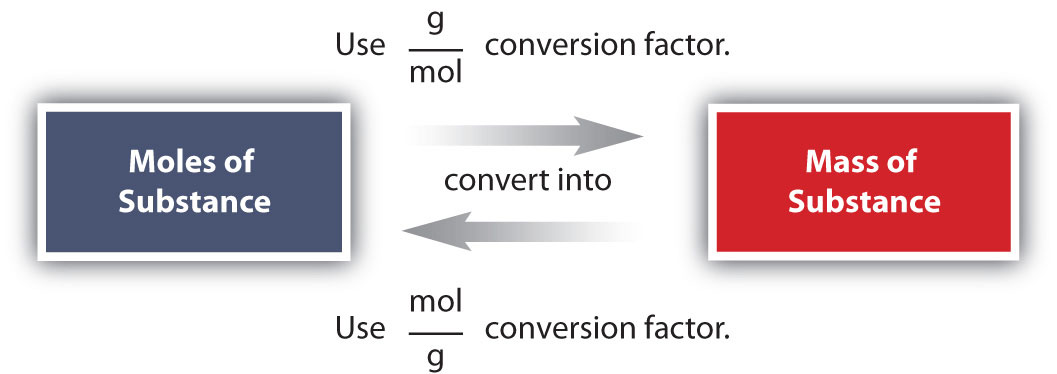
The tooth mass of whatever substance is the mass in grams of ane mole of representative particles of that substance. The representative particles can be atoms, molecules, or formula units of ionic compounds. This relationship is oft used in the laboratory. The simplest blazon of manipulation using molar mass as a conversion factor is a mole-mass conversion (or its reverse, a mass-mole conversion). In such a conversion, nosotros use the tooth mass of a substance as a conversion factor to convert mole units into mass units (or, conversely, mass units into mole units).
We also established that one mol of Al has a mass of 26.98 g (Case). Stated mathematically,
1 mol Al = 26.98 thou Al
We can dissever both sides of this expression by either side to get one of two possible conversion factors:
\[\mathrm{\dfrac{i\: mol\: Al}{26.98\: g\: Al}\, and\, \dfrac{26.98\: yard\: Al}{1\: mol\: Al}} \label{Eq1}\]
The first conversion factor can be used to catechumen from mass to moles, and the second converts from moles to mass. Both tin can be used to solve bug that would be difficult to do "by centre."
Example \(\PageIndex{1}\)
What is the mass of three.987 mol of Al?
Solution
| Steps for Problem Solving | Calculate the mass of 3.987 moles of aluminum (Al). |
|---|---|
| Identify the "given"data and what the problem is asking you lot to "notice." | Given: 3.987 mol of Al Find: g Al |
| List other known quantities | i mol Al = 26.98 yard Al |
| Prepare a concept map and use the proper conversion factor. | 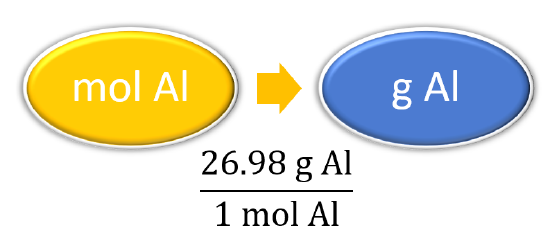 |
| Cancel units and calculate. | \(3.987 \: \cancel{\text{mol} \: \ce{Al}} \times \dfrac{26.98 \: \text{g} \: \ce{Al}}{1 \: \cancel{\text{mol} \: \ce{Al}}} = 107.6 \: \text{g} \: \ce{Al}\) |
| Remember well-nigh your result. | The calculated value makes sense because information technology is almost four times times the mass for 1 mole of aluminum. Our final answer is expressed to four pregnant figures. |
Exercise \(\PageIndex{1}\)
How many moles are present in 100. g of Al? (Hint: yous will have to use the other conversion cistron we obtained for aluminum.)
Answer
three.71 g Al
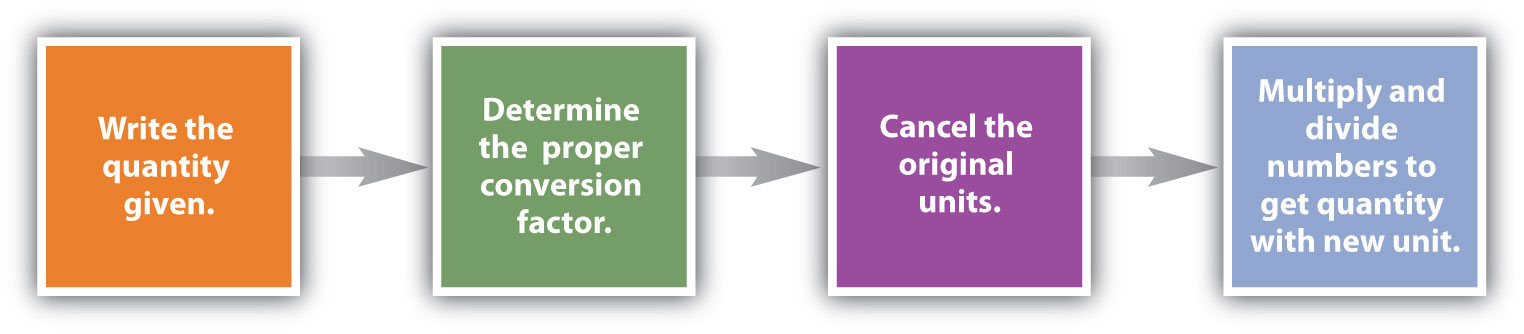
Conversions like this are possible for whatever substance, as long as the proper atomic mass, formula mass, or molar mass is known (or can exist determined) and expressed in grams per mole. Figure \(\PageIndex{1}\) is a chart for determining what conversion factor is needed, and Effigy \(\PageIndex{two}\) is a menstruation diagram for the steps needed to perform a conversion.
Suppose that for a certain experiment you need three.00 moles of calcium chloride \(\left( \ce{CaCl_2} \right)\). Since calcium chloride is a solid, it would be convenient to use a rest to measure the mass that is needed. Dimensional analysis volition allow y'all to calculate the mass of \(\ce{CaCl_2}\) that you lot should measure as show in Example \(\PageIndex{3}\).
Example \(\PageIndex{2}\): Calcium Chloride
Calculate the mass of three.00 moles of calcium chloride (CaCl2).

Solution
| Steps for Problem Solving | Calculate the mass of 3.00 moles of calcium chloride (CaCl2). |
|---|---|
| Place the "given"information and what the problem is asking you to "find." | Given: 3.00 moles of CaCl2 Notice: thousand CaCl2 |
| List other known quantities | 1 ml CaCl2 = 110.98 g CaCltwo |
| Prepare a concept map and use the proper conversion factor. | |
| Cancel units and calculate. | \(3.00 \: \cancel{\text{mol} \: \ce{CaCl_2}} \times \dfrac{110.98 \: \text{g} \: \ce{CaCl_2}}{1 \: \cancel{\text{mol} \: \ce{CaCl_2}}} = 333 \: \text{g} \: \ce{CaCl_2}\) |
| Recall about your event. |
Exercise \(\PageIndex{two}\): Calcium Oxide
What is the mass of \(7.l \: \text{mol}\) of (calcium oxide) \(\ce{CaO}\)?
- Respond:
- 420.sixty g
Example \(\PageIndex{3}\): Water
How many moles are present in 108 grams of water?
Solution
| Steps for Problem Solving | How many moles are nowadays in 108 grams of h2o? |
|---|---|
| Identify the "given"data and what the problem is asking y'all to "find." | Given: 108 chiliad H2O Find: mol H2O |
| List other known quantities | \(i \: \text{mol} \: \ce{H_2O} = 18.02 \: \text{m}\) H2O |
| Set up a concept map and use the proper conversion factor. | |
| Cancel units and summate. | \(108 \: \cancel{\text{g} \: \ce{H_2O}} \times \dfrac{1 \: \text{mol} \: \ce{H_2O}}{18.02 \: \abolish{\text{g} \: \ce{H_2O}}} = 5.99 \: \text{mol} \: \ce{H_2O}\) |
| Think about your result. |
Practice \(\PageIndex{3}\): Nitrogen Gas
What is the mass of \(vii.fifty \: \text{mol}\) of Nitrogen gas \(\ce{N2}\)?
- Answer:
- 210 yard
Mole and Mass Relationships in Chemical Equations
We take established that a balanced chemical equation is balanced in terms of moles likewise as atoms or molecules. Nosotros have used balanced equations to set up ratios, now in terms of moles of materials, that we can use every bit conversion factors to answer stoichiometric questions, such as how many moles of substance A react with so many moles of reactant B. We can extend this technique even farther. Recall that we can relate a molar amount to a mass amount using tooth mass. We can apply that power to answer stoichiometry questions in terms of the masses of a particular substance, in add-on to moles. Nosotros do this using the following sequence:
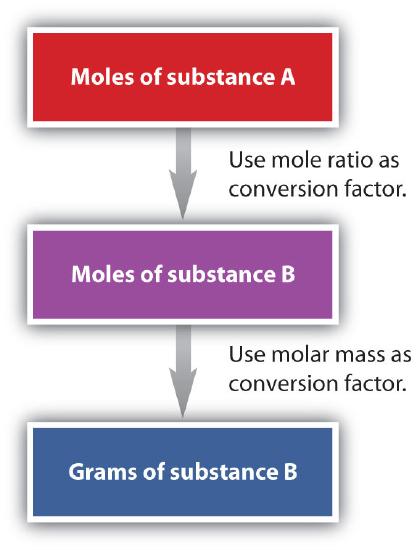
Collectively, these conversions are called mole-mass calculations.
As an instance, consider the balanced chemical equation
\[Fe_2O_3 + 3SO_3 \rightarrow Fe_2(SO_4)_3\]
If we have 3.59 mol of FetwoOthree, how many grams of So3 can react with it? Using the mole-mass adding sequence, nosotros tin make up one's mind the required mass of And then3 in two steps. Beginning, we construct the advisable molar ratio, determined from the balanced chemical equation, to summate the number of moles of SOiii needed. Then using the molar mass of SO3 as a conversion cistron, nosotros determine the mass that this number of moles of Then3 has.
As usual, we starting time with the quantity nosotros were given:
\[\mathrm{3.59\: \cancel{ mol\: Fe_2O_3 } \times \left( \dfrac{3\: mol\: SO_3}{i\: \cancel{ mol\: Fe_2O_3}} \right) =x.77\: mol\: SO_3} \label{Eq2}\]
The mol FetwoO3 units cancel, leaving mol SO3 unit of measurement. At present, we have this answer and catechumen it to grams of SO3, using the molar mass of Then3 as the conversion cistron:
\[\mathrm{10.77\: \bcancel{mol\: SO_3} \times \left( \dfrac{80.06\: g\: SO_3}{1\: \bcancel{ mol\: SO_3}} \right) =862\: g\: SO_3} \label{Eq3}\]
Our last answer is expressed to three significant figures. Thus, in a two-step process, we find that 862 1000 of Theniii will react with 3.59 mol of Atomic number 262O3. Many issues of this type can be answered in this way.
The same two-step problem can also be worked out in a single line, rather than equally two split up steps, as follows:
\[ 3.59 \abolish{\, mol \, Fe_2O_3} \times \underbrace{\left( \dfrac{ 3 \bcancel{ \, mol\, SO_3}}{ 1 \cancel{\, mol\, Fe_2O_3}} \right)}_{\text{converts to moles of Then}_3} \times \underbrace{ \left( \dfrac{ 80.06 {\, g \, SO_3}}{ 1 \, \bcancel{ mol\, SO_3}} \right)}_{\text{converts to grams of SO}_3} = 862\, one thousand\, SO_3\]
We get exactly the aforementioned reply when combining all the math steps together as nosotros practice when we summate one footstep at a fourth dimension.
Example \(\PageIndex{4}\): Generation of Aluminum Oxide
How many moles of HCl will be produced when 249 chiliad of AlCl 3 are reacted according to this chemical equation?
\[2AlCl_3 + 3H_2O(ℓ) → Al_2O_3 + 6HCl(m) \nonumber\]
Solution
| Steps for Trouble Solving | How many moles are in 249 g of AlCl 3 ? |
|---|---|
| Identify the "given"information and what the problem is request yous to "observe." | Given: 249 k AlCl iii Discover: moles HCl |
| List other known quantities | 1 mol AlCl iii = 133.33 g/mol six mol of HCl to 2 mol AlCl3 |
| Fix a concept map and use the proper conversion cistron. | |
| Cancel units and calculate. | \(249\, \cancel{1000\, AlCl_{3}}\times \dfrac{ane\, \abolish{mol\, AlCl_{3}}}{133.33\, \cancel{grand\, AlCl_{3}}}\times \dfrac{six\, mol\, HCl}{two\, \cancel{mol\, AlCl_{three}}}=5.threescore\, mol\, HCl\) |
| Call back about your result. | Since 249 thou of AlCl3 is less than 266.66 g, the mass for 2 moles of AlCl3 and the human relationship is 6 mol of HCl to 2 mol AlCl3 , the answer should be less than six moles of HCl. |
Practise \(\PageIndex{four}\): Generation of Aluminum Oxide
How many moles of Al 2 O 3 volition be produced when 23.9 one thousand of H 2 O are reacted according to this chemic equation?
\[2AlCl_3 + 3H_2O(ℓ) → Al_2O_3 + 6HCl(m) \nonumber\]
- Answer
- 0.442 mol Al ii O 3
Molar Relationships in Chemical Equations
Previously, you learned to balance chemic equations by comparing the numbers of each blazon of atom in the reactants and products. The coefficients in front of the chemical formulas stand for the numbers of molecules or formula units (depending on the type of substance). Hither, we will extend the meaning of the coefficients in a chemical equation.
Consider the simple chemical equation
\[2H_2 + O_2 → 2H_2O\]
The convention for writing balanced chemical equations is to apply the everyman whole-number ratio for the coefficients. Nevertheless, the equation is balanced equally long every bit the coefficients are in a 2:1:two ratio. For case, this equation is as well balanced if we write information technology as
\[4H_2 + 2O_2 → 4H_2O\]
The ratio of the coefficients is 4:2:4, which reduces to ii:ane:ii. The equation is as well balanced if nosotros were to write it as
\[22H_2 + 11O_2 → 22H_2O\]
because 22:11:22 also reduces to 2:1:2.
Suppose we want to use larger numbers. Consider the following coefficients:
\[12.044 \times ten^{23}\; H_2 + 6.022 \times 10^{23}\; O_2 → 12.044 \times 10^{23}\; H_2O\]
These coefficients besides have the ratio ii:i:2 (cheque it and see), and then this equation is balanced. Simply 6.022 × 1023 is ane mol, while 12.044 × 1023 is 2 mol (and the number is written that way to make this more obvious), and then we can simplify this version of the equation by writing information technology as
\[2 \;mol\; H_2 + i\; mol\; O_2 → 2 \;mol\; H_2O\]
Nosotros can go out out the word mol and non write the i coefficient (equally is our habit), so the final form of the equation, notwithstanding balanced, is
\[2H_2 + O_2 → 2H_2O\]
Now nosotros interpret the coefficients every bit referring to tooth amounts, non individual molecules. The lesson? Balanced chemical equations are counterbalanced non merely at the molecular level but besides in terms of molar amounts of reactants and products. Thus, we can read this reaction as "2 moles of hydrogen react with i mole of oxygen to produce two moles of water."
By the same token, the ratios nosotros constructed to draw molecules reaction can also be synthetic in terms of moles rather than molecules. For the reaction in which hydrogen and oxygen combine to make water, for example, nosotros can construct the following ratios:
\[\mathrm{\dfrac{2\: mol\: H_2}{i\: mol\: O_2}\: or\: \dfrac{1\: mol\: O_2}{two\: mol\: H_2}}\]
\[\mathrm{\dfrac{2\: mol\: H_2O}{1\: mol\: O_2}\: or\: \dfrac{ane\: mol\: O_2}{2\: mol\: H_2O}}\]
\[\mathrm{\dfrac{2\: mol\: H_2}{2\: mol\: H_2O}\: or\: \dfrac{2\: mol\: H_2O}{2\: mol\: H_2}}\]
We tin use these ratios to determine what amount of a substance, in moles, will react with or produce a given number of moles of a different substance. The study of the numerical relationships between the reactants and the products in balanced chemical reactions is called stoichiometry.
Example \(\PageIndex{v}\)
How many moles of oxygen react with hydrogen to produce 27.vi mol of H2O?
Solution
| Steps for Problem Solving | How many moles of oxygen react with hydrogen to produce 27.vi mol of H2O? |
|---|---|
| Find a balanced equation that describes the reaction | Unbalanced: H2 + O2 → HtwoO Balanced: 2 Hii + O2 → ii H2O |
| Identify the "given"information and what the problem is asking you to "find." | Given: moles H2O Find: moles oxygen |
| List other known quantities | 1 mol Otwo = ii mol H2O |
| Set up a concept map and use the proper conversion factor. | 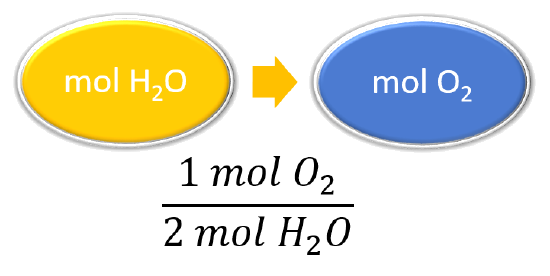 |
| Cancel units and calculate. | \(\mathrm{\abolish{27.half dozen\: mol\: H_2O}\times\dfrac{1\: mol\: O_2}{\cancel{2\: mol\: H_2O}}=xiii.8\: mol\: O_2}\) To produce 27.6 mol of H2O, 13.eight mol of Otwo react. |
| Think about your event. | Since each mole of oxygen produces twice as many moles of water, it makes sense that the produced amount is greater than the reactant amount |
Example \(\PageIndex{6}\)
How many moles of ammonia are produced if 4.20 moles of hydrogen are reacted with an backlog of nitrogen?
Solution
| Steps for Trouble Solving | How many moles of ammonia are produced if iv.twenty moles of hydrogen are reacted with an backlog of nitrogen |
|---|---|
| Detect a balanced equation that describes the reaction | Unbalanced: Ntwo + H2 → NH3 Balanced: Nii + 3 H2 → ii NH3 |
| Identify the "given"information and what the problem is asking you to "observe." | Given: \(\ce{H_2} = 4.20 \: \text{mol}\) Observe: \(\text{mol}\) of \(\ce{NH_3}\) |
| Listing other known quantities | 3 mol H2 = ii mol NHthree |
| Prepare a concept map and use the proper conversion factor. | 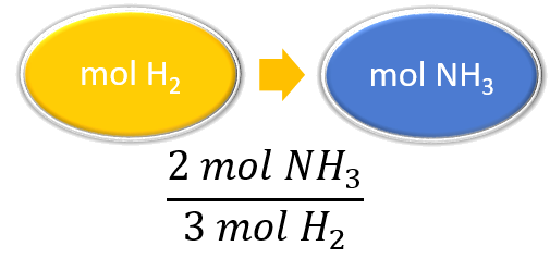 |
| Cancel units and calculate. | \(\abolish{4.20 \: \text{mol} \: H_2} \times \dfrac{2 \: \text{mol} \: NH_3}{\cancel{3 \: \text{mol} \: H_2}} = 2.eighty \: \text{mol} \: NH_3\) The reaction of \(4.20 \: \text{mol}\) of hydrogen with excess nitrogen produces \(2.80 \: \text{mol}\) of ammonia |
| Call back about your result. | The result corresponds to the 3:2 ratio of hydrogen to ammonia from the counterbalanced equation. |
Exercise \(\PageIndex{5}\)
- Given the following balanced chemical equation,\[\ce{C5H12 + 8O2 → 5CO2 + 6H2O}\]how many moles of H2O can be formed if 0.0652 mol of C5H12 were to react?
- Residue the following unbalanced equation and determine how many moles of H2O are produced when 1.65 mol of NHthree react.\[\ce{NH3 + O2 → N2 + H2o} \nonumber\]
- Respond a:
- 3.14 mol H2O
- Answer b:
- 4NHthree + 3O2 → 2N2 + 6HiiO; 2.48 mol HiiO
Mass Relationships in Chemic Equations
It is a modest step from mole-mass calculations to mass-mass calculations. If we start with a known mass of 1 substance in a chemical reaction (instead of a known number of moles), nosotros tin calculate the corresponding masses of other substances in the reaction. The first pace in this case is to convert the known mass into moles, using the substance'southward molar mass as the conversion gene. Then—and simply so—we use the counterbalanced chemical equation to construct a conversion gene to convert that quantity to moles of another substance, which in plow tin be converted to a respective mass. Sequentially, the procedure is as follows:
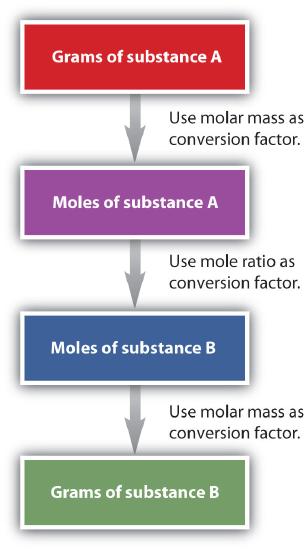
This three-part process can be carried out in three discrete steps or combined into a single adding that contains three conversion factors. The following example illustrates both techniques.
Example \(\PageIndex{7}\): Decomposition of Ammonium Nitrate
Ammonium nitrate decomposes to dinitrogen monoxide and h2o co-ordinate to the following equation.
\[\ce{NH_4NO_3} \left( s \right) \rightarrow \ce{N_2O} \left( g \correct) + 2 \ce{H_2O} \left( 50 \right) \nonumber\]
In a certain experiment, \(45.7 \: \text{g}\) of ammonium nitrate is decomposed. Find the mass of each of the products formed.
| Steps for Problem Solving | Example \(\PageIndex{2}\) |
|---|---|
| Identify the "given"data and what the problem is asking you to "find." | Given: \(45.seven \: \text{chiliad} \: \ce{NH_4NO_3}\) Mass \(\ce{N_2O} = ? \: \text{g}\) Mass \(\ce{H_2O} = ? \: \text{g}\) |
| List other known quantities | 1 mol \(\ce{NH_4NO_3} = 80.06 \: \text{1000/mol}\) ane mol \(\ce{N_2O} = 44.02 \: \text{yard/mol}\) one mol \(\ce{H_2O} = 18.02 \: \text{grand/mol}\) 1 mol NH4NOiii to i mol NiiO to 2 mol HiiO |
| Prepare 2 concept maps and use the proper conversion gene. | |
| Abolish units and summate. | \(45.7 \: \text{g} \: \ce{NH_4NO_3} \times \dfrac{i \: \text{mol} \: \ce{NH_4NO_3}}{lxxx.06 \: \text{thou} \: \ce{NH_4NO_3}} \times \dfrac{1 \: \text{mol} \: \ce{N_2O}}{1 \: \text{mol} \: \ce{NH_4NO_3}} \times \dfrac{44.02 \: \text{grand} \: \ce{N_2O}}{1 \: \text{mol} \: \ce{N_2O}} = 25.1 \: \text{one thousand} \: \ce{N_2O}\) \(45.seven \: \text{g} \: \ce{NH_4NO_3} \times \dfrac{i \: \text{mol} \: \ce{NH_4NO_3}}{80.06 \: \text{g} \: \ce{NH_4NO_3}} \times \dfrac{ii \: \ce{H_2O}}{1 \: \text{mol} \: \ce{NH_4NO_3}} \times \dfrac{xviii.02 \: \text{g} \: \ce{H_2O}}{1 \: \text{mol} \: \ce{H_2O}} = 20.6 \: \text{g} \: \ce{H_2O}\) |
| Call back virtually your upshot. | The total mass of the ii products is equal to the mass of ammonium nitrate which decomposed, demonstrating the police of conservation of mass. Each respond has three significant figures. |
Do \(\PageIndex{6}\): Carbon Tetrachloride
Methane can react with elemental chlorine to make carbon tetrachloride (\(\ce{CCl_4}\)). The balanced chemical equation is as follows.
\[\ce{CH4 (g) + four Cl2 (g) → CCl2 (l) + 4 HCl (fifty) }\]
How many grams of HCl are produced by the reaction of 100.0g of \(\ce{CH4}\)?
- Answer
- 908.7g HCl
Summary
- Calculations involving conversions between moles of a substance and the mass of that substance are described.
- The balanced chemical reaction can be used to decide molar and mass relationships between substances.
Source: https://chem.libretexts.org/Bookshelves/Introductory_Chemistry/Chemistry_for_Changing_Times_(Hill_and_McCreary)/05%3A_Chemical_Accounting/5.04%3A_Molar_Mass-_Mole-to-Mass_and_Mass-to-Mole_Conversions
Posted by: pettypubjewer.blogspot.com


0 Response to "How To Find Molar Mass From Moles"
Post a Comment